Carnot Engine
Carnot Engine: Overview
This Topic covers sub-topics such as Carnot Cycle, Carnot's Engine, Carnot Theorem, Efficiency of Carnot Cycle, Work Done in Compression, Work Done in Expansion, Compression in Carnot Cycle and, Expansion in Carnot Cycle
Important Questions on Carnot Engine
Even Carnot engine cannot give efficiency because we cannot
According to Carnot’s theorem, all heat engines operating between a given constant temperature source and sink, none has a _____ efficiency than a reversible engine.
The efficiency of all reversible heat engines operating between the same heat reservoirs is :
Irrespective of the operation details, every Carnot engine is efficient between two heat reservoirs.
calculate the work done on the gas in carnot cycle during compression.
Isothermal compression in Carnot cycle happened at _____.
The correct sequence of the processes taking place in a carnot cycle is
A carnot engine has an efficiency of .Its efficiency is to be increased to .By what must the temperature of the source be increased if the sink is at ?
The Carnot cycle of a reversible heat engine consists of two isothermal and two _____ processes.
A cyclic heat engine does 50kJ of work per cycle. If efficiency of engine is 75%, the heat rejected per cycle will be
In the Carnot cycle, there are a total of 4 processes which take place?
An engine operates by taking a monoatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to _____.
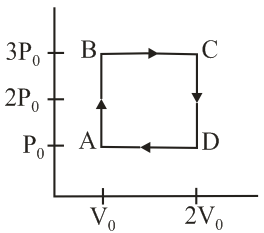
An ideal heat engine exhausting heat at is to have a efficiency. It must take the heat at:
The temperatures and of heat reservoirs in the ideal Carnot engine are and respectively. If increases by , the efficiency of the engine in percentage is
A Carnot engine absorbs of heat energy from a reservoir at and rejects of heat during each cycle, then the temperature of sink is
An engine has an efficiency of . When the temperature of sink is reduced by , its efficiency is doubled. The temperatures of source and sink are,
Two Carnot engines and are operated in series. The first one , receives heat at and rejects a reservoir at temperature . The second engine receives heat rejected by the first engine and, in turn, rejects a heat reservoir at . Calculate the temperature , if the work outputs of the two engines are equal
Efficiency of a Carnot engine working between reservoirs and is
